Structure of Cotton Fiber
Apu Kumar Das
B.Sc. in Textile Technology
Chittagong Textile Engineering College
Email: akdas.textile13@gmail.com
Introduction:
Cotton is a soft, staple fiber that grows in a protective capsule known as boll around the seeds of cotton plant. It is a seed fiber. Cotton is called king of textile fibers. The fiber is spun into yarn and used to make a soft, breathable textile, which is the most widely used form of textile for clothing. The classification of cotton is done on the bases of fineness, staple length, maturity, degree of contamination, and strength. Cotton blends easily with other fibers; mostly with the polyester and viscose. Cotton is used mainly for apparel as well as for home and furnishing textiles. Only 10% of all cotton fibers are processed into technical textiles. Cotton is often used in the manufacture of curtains, tents, and tarpaulins. It is also preferred widely for apparel including blouses, shirts, dresses, children’s wear, active wear, separates, swimwear, underwear, suits, jackets, skirts, pants, sweaters, hosiery, and neckwear. Home fashion articles of cotton are curtains, draperies, bedspreads, sheets, towels, table cloths, table mats, and napkins. Some of the industrial applications of this fiber include ropes, bags, shoes, conveyor belts, filter cloth, medical supplies, etc. Its strength and absorbency make it an ideal fabric for medical textiles such as bandages and swabs. It has low thermal conductivity, and is, therefore, ideal material for both summer and winter clothing. In summer, it prevents the skin from heat, and in winter it preserves the warmth of body.

Structure of Cotton Fiber:
Cotton, the seed hair of plants of the genus Gossypium, is the purest form of cellulose readily available in nature. It has many desirable fiber properties making it an important fiber for textile applications. Cotton is the most important of the raw materials for the textile industry. The structure of a cotton fiber composed of microfibrils, which build up the primary cell wall and the three layers of the secondary cell wall according to a typical orientation. The fineness of cotton fibers varies between 1 and 4 dtex, and the length varies between 10 and 60 mm. Cotton fiber lengths are mostly between 25 and 30 mm. The density is 1.50 to 1.54 g/cm3.
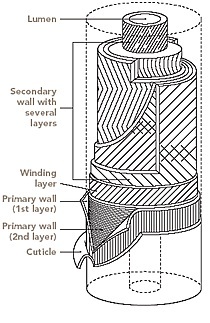
The structure of cotton fiber can be used as a representative case to discuss the general structure of native cellulose fibers as many similarities to the other natural cellulose fibers exist (Figure-2). In dried state the fiber exhibits a characteristic oval shape, which results from the collapse of the fiber during drying of the liquid cell plasma. The inner part of the fiber is filled with cell plasma which dries during the last stage of seed ripening and leaves an empty hollow channel, the so-called lumen. Thus, the fiber is not round, but has a diameter between 12 and 22 μm. The cell wall has a thickness of 2–5 μm, the values being dependent on the quality of the fiber and the status of fiber ripeness.

The outer layer of the fiber is called cuticula and mainly consists of waxes and pectin. The next layer is the primary wall, followed by the secondary wall. Both layers consist of cellulose fibrils and form the structural body of the fiber. Fibers that have been harvested too early exhibit a thinner cell wall and are called unripe fibers. Fibers that exhibit a very thin cell wall have been interrupted in their growth at a very early stage, for example, due to insects and are called “dead” cotton.

The content of immature and dead cotton in the harvested cotton is a relevant quality determining factor, as the dyeing properties of these fibers are substantially different. The lower cellulose content in the cell wall of these fibers leads to lower dyestuff fixation, the fibers appear light and thus reduce the levelness of the dyeing and appear as lighter fibers in a dark fabric.
The cotton fiber is a single biological cell with a multilayer structure. The layers in the cell structure are, from the outside of the fiber to the inside, cuticle, primary wall, secondary wall, and lumen. These layers are different structurally and chemically. The primary and secondary walls have different degrees of crystallinity, as well as different molecular chain orientations. The cuticle, composed of wax, proteins, and pectins, is 2.5% of the fiber weight and is amorphous. The primary wall is 2.5% of the fiber weight, has a crystallinity index of 30%, and is composed of cellulose. The secondary wall is 91.5% of the fiber weight, has a crystallinity index of 70%, and is composed of cellulose. The lumen is composed of protoplasmic residues.

Cotton fiber has a fibrillar structure. The whole cotton fiber contains 88 to 96.5% of cellulose, the rest are non-cellulosic polysaccharides constituting up to 10% of the total fiber weight. The primary wall in mature fibers is only 0.5-1 µm thick and contains about 50% of cellulose. Non-cellulosic constituents consist of pectins, fats and waxes, proteins and natural colorants. The secondary wall, containing about 92- 95% cellulose, is built of concentric layers with alternatic shaped twists. The layers consist of densely packed elementary fibrils, organized into micro fibrils and macro fibrils. They are held together by strong hydrogen bonds. The lumen forms the centre of the fibers. Cotton is composed almost entirely of the polysaccharide cellulose. Cotton cellulose consists of crystalline fibrils varying in complexity and length and connected by less organized amorphous regions with an average ratio of about two-thirds crystalline and one-third non-crystalline material, depending on the method of determination.

Cellulose is one of the main constituent of the cotton fiber. The chemical composition of cellulose is simple, consisting of anhydroglucose units joined by β-1,4-glucosidic bonds to form linear polymeric chains. The chain length, or degree of polymerisation (DP), of a cotton cellulose molecule represents the number of anhydroglucose units connected together to form the chain molecule. DP of cotton may be as high as 14 000, but it can be easily reduced to 1000–2000 by different purification treatments with alkali. The crystalline regions probably have a DP of 200 to 300. Correspondingly, the molecular weight (MW) of cotton usually lies in the range of 50,000–1,500,000 depending on the source of the cellulose. The individual chains adhere to each other along their lengths by hydrogen bonding and Van der Waals forces. The physical properties of the cotton fiber as a textile material, as well as its chemical behavior and reactivity, are determined by arrangements of the cellulose molecules with respect to each other and to the fiber axis.

Non Cellulosic Constituents of Cotton:
The primary wall is about 1 µm thick and comprises only about 1 % of the total thickness of cotton fiber. The major portion of the non-cellulosic constituents of cotton fiber is present in or near the primary wall. Non cellulosic impurities, such as fats, waxes, proteins, pectins, natural colorants, minerals and water-soluble compounds found to a large extent in the cellulose matrix of the primary wall and to a lesser extent in the secondary wall strongly limit the water absorbency and whiteness of the cotton fiber. Pectin is located mostly in the primary wall of the fiber.
It is composed of a high proportion of D-galacturonic acid residues, joined together by α(1→4)-linkages. The carboxylic acid groups of some of the galacturonic acid residues are partly esterified with methanol. Pectic molecule can be called a block-copolymer with alternating the esterified and the non-esterified blocks. In the primary cell wall pectin is covalently linked to cellulose or in other plants to hemicellulose, or that is strongly hydrogen- bonded to other components. Pectin is like powerful biological glue. The mostly water-insoluble pectin salts serve to bind the waxes and proteins together to form the fiber`s protective barrier.
Chemical Composition of Cotton:
The main component of cotton is cellulose, though the precise proportion varies with the source of the cotton and the growing conditions. Also, for a given cotton fiber, the composition differs between the fiber surface and the interior of the fiber. An average composition by percentage of (dry) cotton fiber is shown in below Table.
The general state of knowledge of the chemical composition of a mature cotton fiber is presented in Table.
| Composition of a Fiber | Composition of the Cuticle% | |||
| Constituent | Typical% | Low% | High% | |
| Cellulose | 94.0 | 88.0 | 96.0 | |
| Protein (N-6.25) | 1.3 | 1.1 | 1.9 | 30.4 |
| Pectic substances | 0.9 | 0.7 | 1.2 | 19.6 |
| Wax | 0.6 | 0.4 1 | 1.0 | 17.4 |
| Mineral matters | 1.2 | 0.7 | 1.6 | 6.5 |
| Maleic, citric, and other organic acids | 0.8 | 0.5 | 1.0 | |
| Total sugars | 0.3 | |||
| Cutin | 8.7 | |||
Table shows that non-cellulosic materials account for only a very small amount of the fiber weight. These materials are amorphous and are located in the cuticle and the lumen. The cuticle forms a protective layer to shield the cotton from environmental attacks and water penetration. Waxy materials are mainly responsible for the non-absorbent characteristics of raw cotton. Pectins may also have an influence, since 85% of the carboxyl groups in the pectins are methylated.
Row cotton fibers have to go through several chemical processes to obtain properties suitable for use. With scouring, non-cellulose substances (wax, pectin, proteins, hemicelluloses…) that surround the fiber cellulose core are removed, and as a result, fibers become hydrophilic and suitable for bleaching, dyeing and other processing.
By removing pectin, it is easier to remove all other non-cellulosic substances. The processes of bioscouring that are in use today are based on the decomposition of pectin by the enzymes called pectinases.
References:
- Textile Chemistry by Thomas Bechtold, Tung Pham
- The Chemistry of Textile Fibres, 2nd Edition by Robert R. Mather, Roger H. Wardman
- Textile Engineering-An Introduction Edited by Yasir Nawab
- Textile Technology-An Introduction, 2nd Edition by Thomas Gries, Dieter Veit, Burkhard Wulfhorst
You may also like:
- Cotton: The King of Textile Fibers | Cultivation to Production
- Technical Properties of Cotton Fiber
- Chemical Composition of Cotton Fiber
- Preparatory Process of Cotton | Preparatory Process of Cellulosic Fiber
Founder & Editor of Textile Learner. He is a Textile Consultant, Blogger & Entrepreneur. Mr. Kiron is working as a textile consultant in several local and international companies. He is also a contributor of Wikipedia.






